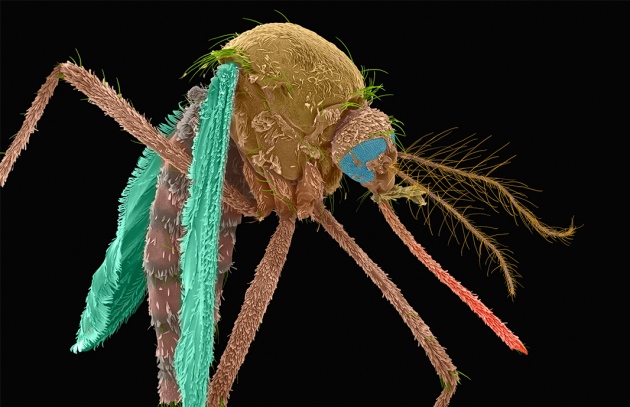
After a decade of fighting for regulatory approval and public acceptance, a biotechnology firm has released genetically engineered mosquitoes into the open air in the United States for the first time. The experiment, launched this week in the Florida Keys – over the objections of some local critics – tests a method for suppressing populations of wild Aedes aegypti mosquitoes, which can carry diseases such as Zika, dengue, chikungunya and yellow fever.
Oxitec, the firm based in Abingdon, UK, that developed the mosquitoes, has previously field-tested the insects in Brazil, Panama, the Cayman Islands and Malaysia.
But until now, owing to a circuitous series of regulatory decisions and pushback from Florida residents, no genetically engineered mosquito had been trialled in the United States – even though the country previously allowed tests of a genetically engineered diamondback moth (Plutella xylostella) in New York and an engineered pink bollworm (Pectinophora gossypiella) in Arizona, both developed by Oxitec.
“When something new and revolutionary comes along, the immediate reaction of a lot of people is to say: ‘Wait’,” says Anthony James, a molecular biologist focused on bioengineered mosquitoes at the University of California, Irvine. “So, the fact that [Oxitec] was able to get the trial on the ground in the United States is a big deal.”
Aedes aegypti makes up about for pe cent of the mosquito population in the Keys, a chain of tropical islands off the southern tip of Florida. But it is responsible for practically all mosquito-borne disease transmitted to humans in the region, according to the Florida Keys Mosquito Control District (FKMCD), which is working closely with Oxitec on the project.
Researchers and technicians working on the project will release bioengineered male Aedes aegypti mosquitoes, which don’t bite, to mate with the wild female population, responsible for biting prey and transmitting disease. The genetically engineered males carry a gene that passes to their offspring and kills female progeny in early larval stages.
Male offspring won’t die but instead will become carriers of the gene and pass it to future generations. As more females die, the Aedes aegypti population should dwindle.
FKMCD in 2010 approached Oxitec about testing its approach in the Keys, because Florida was – and still is – experiencing an increase in mosquito-borne disease. In 2009, the state began seeing cases of locally transmitted dengue, and, a few years later, locally transmitted Zika.
In late April of this year, project researchers placed boxes containing Oxitec’s mosquito eggs at six locations in three areas of the Keys. The first males are expected to emerge within the first two weeks of May. About 12,000 males will exit the boxes each week over the next 12 weeks. In a second phase later this year, intended to collect even more data, nearly 20 million mosquitoes will emerge over a period of about 16 weeks, according to Oxitec.
Genetically engineered mosquitoes are an alternative to insecticides, which are used heavily in the United States to control insect populations. This has resulted in the evolution of mosquitoes that are resistant to insecticides.
Unfortunately, we’re seeing our toolbox shrinking due to resistance,” said Andrea Leal, executive director of FKMCD, at a press conference last week. “That’s one of the reasons why we’re really looking at these new innovative tools and new ways to control this mosquito.”
To monitor the trial’s progress, researchers will use capture devices to trap mosquitoes for study. They will measure how far the male mosquitoes travel from the boxes, how long they live, how effectively they squelch the wild female mosquito population and whether all of the females with the gene are indeed dying. Oxitec mosquitoes carry a fluorescent marker gene that makes them glow when exposed to a specific colour of light, which makes identification easier.
The biotech firm plans to present the results to the US Environmental Protection Agency (EPA), which gave the green light for the trial. The data will help the EPA to determine whether Oxitec can release the mosquitoes more broadly in the United States. The company is still testing them in Brazil and other countries.
Opposition to the Florida field trial has been fierce from some residents in the Keys. Worried about being bitten by the mosquitoes or that the insects will disrupt the Florida ecosystem — and generally unhappy about being chosen as a test site — some have threatened to derail the experiments by spraying insecticides near the release points.
“As you can imagine, emotions run high and there are people who feel really strongly either for or against it,” says molecular biologist Natalie Kofler, who lectures at Harvard Medical School in Cambridge, Massachusetts, and is the founder of Editing Nature, an organisation that advocates for responsible development and oversight of gene-editing technologies. “And I can see how, if you didn’t agree to this, it could be really concerning to have mosquitoes released in your neighbourhood.”
Many of the concerns stem from the uncertainty of a new technology, says Kofler, who has been following this project for years. Oxitec has been engaging with the Florida Keys community to provide answers to queries.
They explained, for instance, the very low likelihood that female mosquitoes with the lethal gene could reproduce. But many people don’t have confidence in what they’re hearing, because it’s coming from a company, says Kofler.
Kofler is hoping that enough data are gathered to assess the mosquitoes’ impact, including on other species in the Keys and local ecosystems and that it’s done “in a way that’s transparent, and in a way that can make some community members feel better about the whole situation.”
Oxitec employees have taken precautions against vandalism by placing their mosquito boxes on private, fenced-in properties, and not disclosing their precise locations to the public.
Oxitec has faced regulatory assessments from three different US federal agencies and opposition from Florida residents over the past decade as it sought approval to release its experimental mosquitoes in the United States for the first time.
March 2010: Oxitec submits a request to the US Department of Agriculture (USDA) to run a field trial with its genetically modified mosquitoes.
October 2011: The USDA says it doesn’t have regulatory jurisdiction over Oxitec’s mosquitoes.
November 2011: The US Food and Drug Administration (FDA) claims jurisdiction over regulating the mosquitoes, so Oxitec submits an application to the agency for a trial in Key Haven, Florida.
August 2016: The FDA approves the trial. The start date depends on the Florida Keys Mosquito Control District (FKMCD) board’s approval of mosquito-release locations.
November 2016: Key Haven residents vote against the trial in a referendum, but elsewhere in Monroe Country, Florida, enough residents vote in favour of it to keep the project afloat.
October 2017: The FDA transfers jurisdiction of Oxitec’s mosquitoes to the US Environmental Protection Agency (EPA).
March 2019: Oxitec transitions to a second-generation mosquito because of advances in technology and requests from the EPA an experimental permit to conduct field trials in Monroe County.
April 2020: The EPA green-lights the project.
August 2020: The FKMCD board votes to proceed with the trial.
April 2021: The trial begins as boxes of genetically engineered mosquitoes are placed in Monroe County’s Cudjoe Key, Ramrod Key and Vaca Key.
- A Nature magazine report











