US considers buying two million shots use in low and middle income countries hard-hit by HIV
There are more than 30,000 new HIV infections in the US every year and 1.2 million people are living with the virus. Worldwide there are 1.3 million new infections annually and nearly 40 million people living with the virus.
HIV response: Lenacapavir approved in Kenya and is set for from nest January
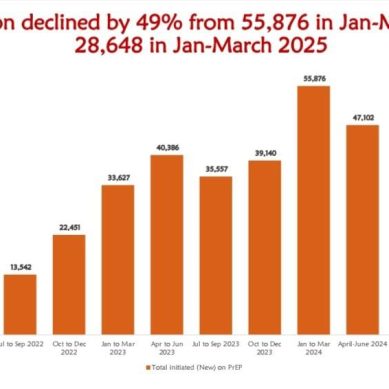
PrEP initiation refers to the process of starting someone on medication to prevent HIV infection before they are exposed to the virus. This typically involves assessing an individual’s risk factors, confirming they are HIV-negative and then prescribing and educating them on the appropriate PrEP regimen.
HIV/Aids: Kenya to start administering newly approved Lenacapavir antiretroviral drug in January
Lenacapavir received approval from the US Food and Drug Administration (FDA) in June 2025 and has been subsequently endorsed in the updated World Health Organization (WHO) guidelines on long-acting HIV prevention, released in July 2025.