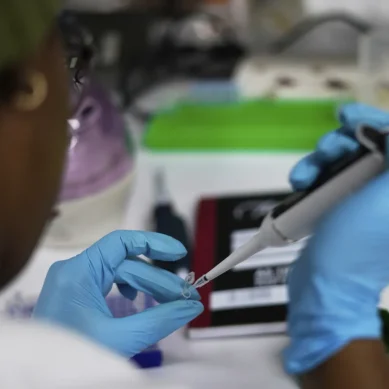
HIV/Aids: Kenya to start administering newly approved Lenacapavir antiretroviral drug in January
Lenacapavir received approval from the US Food and Drug Administration (FDA) in June 2025 and has been subsequently endorsed in the updated World Health Organization (WHO) guidelines on long-acting HIV prevention, released in July 2025.
While Africa has been vital to development of HIV medication, Trump’s funding cuts to UN threatens vaccine research
Significant advances have included clinical trials for lenacapavir, the world’s only twice-a-year shot to prevent HIV, recently approved for use by the US Food and Drug Administration. One study to show its efficacy involved young South Africans.